Does Water Have a Low or High Specific Heat
According to Georgia State University water has the highest specific heat of any common substance. Water had three rotational degrees of freedom.

Heat Capacity Of Water Overview Importance Expii
6 Why does water have a strong surface tension and why is this important.

. Specific heat capacity is heat capacity per gram. However the temperature of liquid water rises and falls more slowly than those of most other liquids. 3 Why does water have more surface tension than other liquids.
For instance the specific heat capacity of water is about five times greater than that of sandThe land cools faster than the sea once the sun goes down and the slow-cooling water can release heat to nearby land during the night. Because it is one of the lightest non-linear molecules. The specific heat often varies with temperature and is different for each state of matter.
For instance if water and copper were both heated by 1 degree Celsius it would require more heat to increase the waters temperature than to heat the. That means it needs to absorb a lot of energy before its temperature changes. Non-linear molecule - all 3 rotational degrees of freedom contribute to the specific heat.
2464 J mol K The heavier elements contain fewer atoms to absorb the energy per gram of material and therefore tend to have lower specific heat capacities. The specific heat capacity from a statistical mechanics point of view is basically the capacity to store energy in certain energy levels translational rotational vibrational electronic. The temperature of water increases as it absorbs heat and decreases as it releases heat.
Once the hydrogen bonds in a water molecule are heated up enough to break the. The specific heat of the water as a liquid is high. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree Celsius C.
4 Why does water have high surface tension but low viscosity. Low molecular mass - so more moles per kg. So a high value means that it takes MORE energy to raise or lower its temperature.
While you can pick a metal tray up a few minutes of it coming. Al 243 JK-mol Cu 245 JK-mol and not very different from air eg. Specific heat is defined as the ability of something to increase in 1 degree celsius.
This is why water is valuable to industries and in your cars radiator as a coolant. Specific heat capacity is defined as the amount of heat required per unit mass to increase the. This means that it takes more heat to raise the temperature of water.
Liquid water has one of the highest specific heats among common substances about 4182 JKkg at 20 C. Anything with a low density will have fewer degrees of freedom per gram by definition and therefore a lower specific heat. Water covers around 70 of the Earths surface and its high specific heat plays a very important role as it is able to absorb a lot of heat without a significant rise in the temperature.
Water has a fairly high specific heat when compared to many other materials. Because of its high heat capacity water can minimize changes in temperature. It makes water an ideal liquid for removing heat from things like the engine of a car.
When these energy levels are low the minimum temperatures required. Because the majority of heat energy is concentrated on breaking the hydrogen bonds the water molecule itself heats up after the bonds are broken. Water has a very high specific heat.
The reason why water has a high specific heat is because there is a large number of hydrogen bonds. The heated water will contribute much of the heat to loosening bending or breaking the hydrogen bonds. A low value means that it does not take very much energy to heat or cool it.
Water has a high specific heat meaning it takes more energy to increase the temperature of water compared to other substances. If you compare molar heat capacities then Al is about the same as any other metal eg. Metals tend to have a low specific heat and water has a very high specific heat.
But that of ice just below 0 C is only 2093 JKkg. Water has a high heat capacity because a lot of heat energy is required to break the hydrogen bonds found in a molecule of water. This will result in a higher heat capacity.
Thus most organisms that live in water would not survive if water had a low specific heat due to the burning effect of the water temperaturespecific heat is the amount of heat it takes to raise a substance to rise 1C. 5 What characteristic allows water to have a high surface tension. The specific heat capacity of a substance is the amount of energy required to raise the temperature of 1 kg of the substance by 1C.
What is heat capacity Example. It may be best to answer by using an example with a high specific heat. Another factor is that a thin metal tray coming out of the oven is going to cool quicker than a thicker heavier glass dish.
Specific heat is J g o K. Hydrogen bonds are considerably harder to break compared to molecule with no hydrogen bonding because of the interactions require higher heat to be broken. N2 21 JK.
A specific heat is the amount of heat or energy required to raise the temperature of a substance by 1 degree Celsius. Although water has the highest specific heat of any common material it is a lot less dense than metals so while a litre of water weighing 1 kgL with a specific heat of 4182 Jkg C holds 4182 jouleL steel with a density of approx 8 kgL depending on alloy and a specific heat of 490 Jkg C holds 3920 JL so if the temperature feeling was caused by heat capacity. How is this useful.
Adding heat to a low specific heat compound will increase its temperature much more quickly than adding heat to a high specific heat compound. Sand and asphalt on the other hand have lower specific heats. 7 What causes the high surface tension low vapor pressure and high boiling point.
And we do indeed see a significantly higher specific heat capacity for water than for compounds analogous to water. In addition to vibration rotations happen a lot to water molecules.
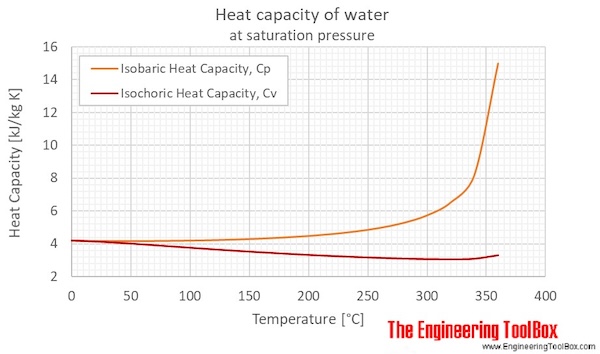
Water Specific Heat Vs Temperature
Specific Heat Heat Of Vaporization And Density Of Water Article Khan Academy
No comments for "Does Water Have a Low or High Specific Heat"
Post a Comment